Advertisements
Advertisements
प्रश्न
Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give reason for your answer.
\[\ce{\underset{I}{CH2 = CH - CH = O} <-> \underset{II}{\overset{⊕}{C}H2 - CH = CH - \overset{Θ}{O}}}\]
उत्तर
The structure I will be more stable than structure II because all the atoms are having complete octet in structure I and the carbon atom having positive charge don’t have a complete octet in structure II.
APPEARS IN
संबंधित प्रश्न
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5NO2
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
CH3CH = CHCHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5 – CHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{C6H5 - \overset{+}{C}H2}\]
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{CH3CH = CH\overset{+}{C}H2}\]
Draw the possible resonance structures for \[\ce{CH3 - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - \overset{+}{C}H2}\] and predict which of the structures is more stable. Give reason for your answer.
The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation.

Draw the resonance structure of the following compounds;
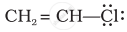
Draw the resonance structure of the following compounds;
CH2 = CH – CH = CH2
Draw the resonance structure of the following compounds;
\[\begin{array}{cc}
\ce{CH2 = CH - C = O}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\]
