Advertisements
Advertisements
प्रश्न
State the inference drawn from the following observations:
Salt S is prepared by reacting dilute sulphuric acid with copper oxide. Identify S.
उत्तर
Salt S is prepared by reacting dilute sulphuric acid with copper oxide. Hence, salt S is copper sulphate.
APPEARS IN
संबंधित प्रश्न
Write a balanced chemical equation for the following:
Action of concentrated sulphuric acid on Sulphur.
State the conditions required for the given reaction to take place:
Any two conditions for the conversion of sulphur dioxide to sulphur trioxide
Name the products formed when hot and concentrated sulphuric acid reacts with Sugar.
Give reason for the following:
Cotton clothes get burnt with concentrated sulphuric acid.
Copy and complete the following table.
Column 3 has the names of gases to be prepared using the substance you enter in column 1, along with dilute or concentrated sulphuric acid, as indicated in column 2.
| Column 1 | Column 2 | Column 3 |
| Substance reacted with acid | Dilute or concentrated sulphuric acid | Gas |
| Hydrogen | ||
| Carbon dioxide | ||
| Only chlorine |
Write the equation for the laboratory preparation of :
(i) Sodium sulphate using dilute sulphuric acid.
(ii) Lead sulphate using dilute sulphuric acid.
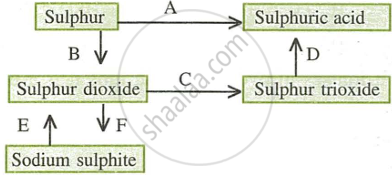
- Name the catalyst which helps in the conversion of sulphur dioxide to sulphur trioxide in step C.
- In the contact process for the manufacture of sulphuric acid, sulphur trioxide is not converted to sulphuric acid by reacting it with water. Instead a two-step procedure is used. Write the equations for the two steps involved in D.
- What type of substance will liberate sulphur dioxide from sodium sulphite in step E?
- Write the equation for the reaction by which sulphur dioxide is converted to sodium sulphite in step F.
Name the gas evolved in following case:
The gas produced by the action of concentrated sulphuric acid on sodium chloride.
Give one equation to show the following property of sulphuric acid:
Acidic nature
Give a balanced chemical equation for the action of sulphuric acid of the following:
Sulphur
Identify the salts P and Q from the observation given below:
On performing the flame test salt P produces a lilac coloured flame and its solution gives a white precipitate with silver nitrate solution. Which is soluble in ammonium hydroxide solution.
