Advertisements
Advertisements
प्रश्न
The amount of heat energy required to melt a given mass of a substance at its melting point without any rise in its temperature is called as the ______.
पर्याय
specific heat capacity
specific latent heat of fusion
latent heat of fusion
specific latent heat of freezing
उत्तर
The amount of heat energy required to melt a given mass of a substance at its melting point without any rise in its temperature is called as the latent heat of fusion.
APPEARS IN
संबंधित प्रश्न
A refrigerator converts 100g of water at 20℃ to ice at – 10℃ in 73.5 min. Calculate the average rate of heat extraction in watt. The specific heat capacity of water is 4.2 J kg-1 K-1, specific latent heat of ice is 336 J g-1 and the specific heat capacity of ice is 2.1 J kg-1 K-1.
What do you mean by the statement?
'The specific latent heat capacity of fusion of ice is 336 J per g'?
Define the following terms:
(i) Specific latent heat,
(ii) Specific latent heat of fusion.
A substance changes from its solid state to the liquid state when heat is supplied to it. What name is given to heat absorbed by the substance.
What happens to the heat supplied to a substance when the heat supplied causes no change in the temperature of the substance?
When 1 g of ice at 0 °C melts to form 1 g of water at 0 °C then, is the latent heat absorbed by the ice or given out by it?
Why does weather become pleasant when it starts freezing in cold countries?
The specific latent heat of vaporisation of steam is 2260 J/g. Comment on this.
Explain, why no tracks are left on the ice during ice skating?
What do you understand by the ‘latent heat of vaporization’ of a substance?
If pressure increases, the melting point of a substance ______.
Define boiling point of a liquid.
Match the columns.
| Column A | Column B |
| 1) Specific latent heat of fusion | a) Air saturated with vapour |
| 2) Specific latent heat of vaporisation | b) Solid converts into liquid |
| 3) Dew point temperature | c) liquid converts into gas |
1 kg of dry air at a temperature of 40 °C can hold a maximum of 49 g of water vapour.
Write scientific reason.
Use a pressure cooker to cook food in cold air.
Observe the following graph and answer the following questions:

- What does the graph represent?
- What does the line AB represent?
- What does the line BC represent?
The diagram below shows a cooling curve for a substance:
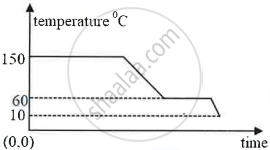
- State the temperatures at which the substance condenses.
- The temperature range in which the substance is in liquid state.
- Why do we prefer ice to ice-cold water for cooling a drink?
