Advertisements
Advertisements
प्रश्न
The boiling point of a 0.2 m solution of a non-electrolyte in water is ______.
(Kb, for water = 0.52 K kg mol−1)
पर्याय
100°C
100.52°C
100.104°C
l00.26°C
MCQ
रिकाम्या जागा भरा
उत्तर
The boiling point of a 0.2 m solution of a non-electrolyte in water is 100.104°C.
Explanation:
Molality of solution = 0.2 m
Kb of water = 0.52 K kg mol−1
Boiling Point Elevation
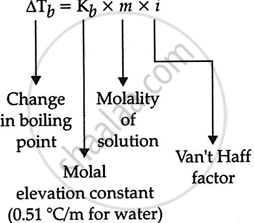
For most non-electrolytes dissolved in water, the van't Hoff factor is essentially 1.
Hence, Elevation in Boiling point = 0.52 × 0.2 = 0.104°C
∴ Therefore, Boiling point = 100 + 0.104
= 100.104°C
shaalaa.com
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
