Advertisements
Advertisements
प्रश्न
The metal complex ion that is paramagnetic is ______.
(Atomic number of Fe = 26, Cu = 29, Co = 27 and Ni = 28)
पर्याय
[Fe(CN)4]2−
[Co(NH3)6]3+
[Ni(CN4)]2−
[Cu(NH3)4]2+
उत्तर
The metal complex ion that is paramagnetic is [Cu(NH3)4]2+.
Explanation:
Paramagnetic substances have unpaired electrons, while diamagnetic substances have all electrons paired
- [Fe(CN)4]2−
Assume x is the oxidation number of Fe in given complex.
x + 4(−1) = −2
x − 4 = − 2
x = − 2 + 4
= + 2
Electronic configuration of Fe = [Ar]3d64s2
Fe+2 = [Ar]3d6
All electrons are paired; hence, it is diamagnetic in nature [Co(NH3)6]3+ - Assume x is the oxidation number of Co in given complex.
x + 6(0) = + 3
x = + 3
Electronic configuration of Co = [Ar] 3d74s2
Co+3 = [Ar]3d6 - All electrons are paired; hence, it is diamagnetic [Ni(CN4)]2−
Assume x is the oxidation number of Ni in the given complex.
x + 4(−1) =− 2
x − 4 = − 2
x = + 2
Electronic configuration of Ni = [Ar] 3d84s2
Ni2+ = [Ar]3d8 - All electrons are paired, hence, it is diamagnetic [Cu(NH3)4]2+.
Assume x is the oxidation number of Cu in the given complex.
x + 4(0) = + 2
x = + 2
Electronic configuration of Cu = [Ar] 3d10 4s1
Cu−2 = [Ar]3d9
It has one unpaired electron; hence, it is paramagnetic.
APPEARS IN
संबंधित प्रश्न
Explain why:
(i) Transition elements form coloured compounds.
(ii) Interhalogen compounds are more reactive than their constituent elements.
(iii) Cu+ is diamagnetic but Cu2+ is paramagnetic. (Z = 29)
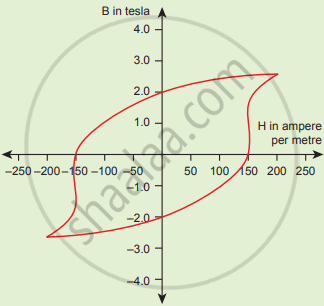
The BH curve for a ferromagnetic material is shown in the figure. The material is placed inside a long solenoid which contains 1000 turns/cm. The current that should be passed in the solenonid to demagnetize the ferromagnet completely is
Example of ferromagnetic substance is ____________.
All those atoms or molecules which have an odd number of electrons are
When heated to high temperature, ferromagnetic substance changes to ____________.
Which of the following statements are correct?
(i) Ferrimagnetic substances lose ferrimagnetism on heating and become paramagnetic.
(ii) Ferrimagnetic substances do not lose ferrimagnetism on heating and remain ferrimagnetic.
(iii) Antiferromagnetic substances have domain structures similar to ferromagnetic substances and their magnetic moments are not cancelled by each other.
(iv) In ferromagnetic substances all the domains get oriented in the direction of magnetic field and remain as such even after removing magnetic field.
Which one of the following homo-diatomic molecule is paramagnetic?
The correct order of bond strength is ______.
The susceptibility of a paramagnetic material is 99. The permeability of the material in Wb/A-m is ______.
[permeability of the free space μ0 = 4π × 10-7 Wb/A - m]
Among the following ions, which one has the highest paramagnetism?
