Advertisements
Advertisements
प्रश्न
Vulcanisation makes rubber:
(i) more elastic
(ii) soluble in inorganic solvent
(iii) crystalline
(iv) more stiff
उत्तर
(i) more elastic
(iv) more stiff
Explanation:
Vulcanisation makes rubber more elastic and stiff. On vulcanization sulphur forms cross-links at the reactive sites of double bonds or at their reactive allylic position and thus rubber gets stiffened.
APPEARS IN
संबंधित प्रश्न
What is natural rubber?
(A) Cis-1,4-polyisoprene
(B) Neoprene
(C) Trans-1,4-polyisoprene
(D) Butyl rubber
How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Identify the monomer in the following polymeric structures.
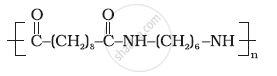
Write the formulae of the raw materials used for preparation of Buna-S
Write the structures of monomers used the following polymers:
Buna S
Complete the following statement by selecting the correct alternative from the choices given:
Natural rubber is a : _________
Which of the following are example of synthetic rubber?
(i) Polychloroprene
(ii) Polyacrylonitrile
(iii) Buna-N
(iv) cis-polyisoprene
Identify the polymer given below:
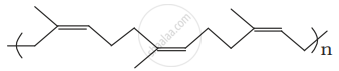
Match materials given in Column I with the polymers given in Column II.
| Column I | Column II |
| (i) Natural rubber latex | (a) Nylon |
| (ii) Wood laminates | (b) Neoprene |
| (iii) Ropes and fibres | (c) Dacron |
| (iv) Polyester fabric | (d) Melamine formaldehyde resins |
| (v) Synthetic rubber | (e) Urea-formaldehyde resins |
| (vi) Unbreakable crockery | (f) cis-polyisoprene |
Match List I with List II.
| List I | List II |
| (Monomer Unit) | (Polymer) |
| (a) Caprolactum | (i) Natural rubber |
| (b) 2-Chloro-1, 3-butadiene | (ii) Buna-N |
| (c) Isoprene | (iii) Nylon-6 |
| (d) Acrylonitrile | (iv) Neoprene |
Choose the correct answer from the options given below:
