Advertisements
Advertisements
प्रश्न
(a) What are the terms defined by the following?
(i) A salt containing a metal ion surrounded by other ions or molecules.
(ii) A base which is soluble in water.
(b) Making use only of substances chosen from those given below:
Dilute sulphuric acidSodium Carbonate
Zinc Sodium sulphite
Lead Calcium carbonate
Give equations for the reactions by which you could obtain :
(i) Hydrogen
(ii) Sulphur dioxide
(iii) Carbon dioxide
(iv) Zinc carbonate (two steps required)
उत्तर
(i) Complex salts
(ii) Alkali
(i) \[\ce{Zn + H2SO4 → ZnSO4 + H2}\]
(ii) \[\ce{Na2SO3 → Na2O + SO2}\]
(iii) \[\ce{Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2)}\]
(iv) \[\ce{Zn + H2SO4 → ZnSO4 + H2}\]
\[\ce{ZnSO4 + Na2CO3 → Na2SO4 + ZnCO3}\]
APPEARS IN
संबंधित प्रश्न
Give balanced chemical equation to prepare the following salt:
Copper chloride using copper carbonate
Write the balanced equation for the preparation of the following salt in the laboratory:
A soluble sulphate by the action of an acid on an insoluble base.
Write the balanced equation for the preparation of the following salt in the laboratory:
An insoluble salt by the action of an acid on another salt.
Write the balanced equation for the preparation of the following salt in the laboratory:
An insoluble base by the action of a soluble base on a soluble salt.
Write the balanced equation for the preparation of the following salt in the laboratory:
A soluble sulphate by the action of an acid on a metal.
Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain: Sodium sulphate
| Dilute sulphuric acid | Copper | Copper carbonate |
| Iron | Sodium carbonate | |
| Sodium | ||
| Zinc |
Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain: Copper sulphate.
| Dilute sulphuric acid | Copper | Copper carbonate |
| Iron | Sodium carbonate | |
| Sodium | ||
| Zinc |
Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain: lron(II) sulphate
| Dilute sulphuric acid | Copper | Copper carbonate |
| Iron | Sodium carbonate | |
| Sodium | ||
| Zinc |
Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain: Zinc carbonate.
| Dilute sulphuric acid | Copper | Copper carbonate |
| Iron | Sodium carbonate | |
| Sodium | ||
| Zinc |
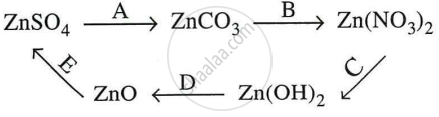
Give equations for the following conversions A to E.