Advertisements
Advertisements
प्रश्न
What is the correct order of decreasing stability of the following cations.
| I. | \[\ce{CH3 - \overset{⊕}{C}H - CH3}\] |
| II. | \[\ce{CH3 - \overset{⊕}{C}H - OCH3}\] |
| III. | \[\ce{CH3 - \overset{⊕}{C}H - CH2 - OCH3}\] |
पर्याय
II > I > III
II > III > I
III > I > II
I > II > III
उत्तर
II > I > III
Explanation:
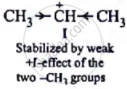
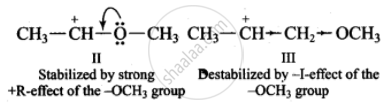
Thus, the stability of carbocations decreases in the order: II > I > III.
APPEARS IN
संबंधित प्रश्न
For the following bond cleavages, use curved arrows to show the electron flow and classify homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
\[\ce{CH3O - OCH3 -> CH3\overset\bullet{\text{O}} + \overset\bullet{\text{O}}CH3}\]
For the following bond cleavages, use curved-arrows to show the electron flow and classify as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
For the following bond cleavages, use curved-arrows to show the electron flow and classify as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
For the following bond cleavages, use curved-arrows to show the electron flow and classify as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
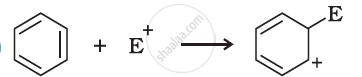
Which of the following carbocation is most stable?
Covalent bond can undergo fission in two different ways. The correct representation involving a heterolytic fission of CH3 – Br is:
Write structures of various carbocations that can be obtained from 2-methylbutane. Arrange these carbocations in order of increasing stability.
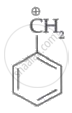 |
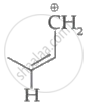 |
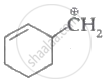 |
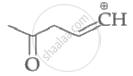 |
| (I) | (II) | (III) | (IV) |
Among the given species the resonance stabilised carbocations are:
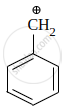 |
\[\ce{CH2 = \overset{⊕}{C}H}\] | \[\ce{CH3 - \overset{⊕}{C}H2}\] | \[\ce{HC ≡ \overset{⊕}{C}}\] |
| A | B | C | D |
The correct order of stability of given carbocation is:
A solution of (–) – 1 – chloro–1–phenylethane in toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of ______.
