Advertisements
Advertisements
प्रश्न
Which of the following graphs is not correct for ideal gas?
पर्याय
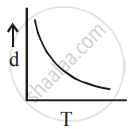
[d = density, P = pressure, T = Temperature]
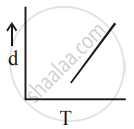
[d = density, P = pressure, T = Temperature]
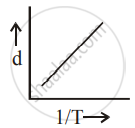
[d = density, P = pressure, T = Temperature]
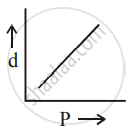
[d = density, P = pressure, T = Temperature]
MCQ
उत्तर

[d = density, P = pressure, T = Temperature]
Explanation:
From the ideal gas equation, we get,
PM = dRT
⇒ d = `["PM"/"R"]1/"T"`
So graph d vs T is not a straight line.
shaalaa.com
Ideal Gas Equation
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
