Advertisements
Advertisements
प्रश्न
Which of the following is correct for P4 molecule of white phosphorus?
(i) It has 6 lone pairs of electrons.
(ii) It has six P–P single bonds.
(iii) It has three P–P single bonds.
(iv) It has four lone pairs of electrons.
उत्तर
(ii) It has six P–P single bonds.
(iv) It has four lone pairs of electrons.
Explanation:
Structure of P4molecule can be represented as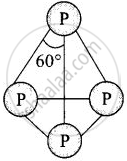
It has total four lone pairs of electrons situated at each P-atom.
It has six P – P single bond.
APPEARS IN
संबंधित प्रश्न
Out of white phosphorus and red phosphorus, which one is more reactive and why?
What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2?
Give reasons for the following:
Red phosphorus is less reactive than white phosphorus.
Answer the following question.
Write the disproportionation reaction of H3PO3.
Which allotrope of phosphorous is most stable?
At what temperature white phosphorus changes to red phosphorus?
What is the spontaneous ignition temperature of white phosphorous?
All elements of Group 15 show allotropy except ______.
Which of the following elements does not show allotropy?
Which one of the following is formed (mainly) when red phosphorus is heated in a sealed tube at 803 K?
