Advertisements
Advertisements
प्रश्न
Which of the following reactions takes place at the anode during the electroplating of an article with silver?
पर्याय
\[\ce{Ag - 1e^{-} -> Ag^{1+}}\]
\[\ce{Ag + 1e^{-} -> Ag^{1-}}\]
\[\ce{Ag - 1e^{-} -> Ag}\]
None of the above
MCQ
उत्तर
\[\ce{Ag - 1e^{-} -> Ag^{1+}}\]
Explanation:
Because oxidation take place at the anode, e– loss takes place there.
shaalaa.com
Examples of Electrolysis - Electrolysis of Copper Sulphate Solution Using Platinum Anode and Copper Or Platinum Cathode
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
APPEARS IN
संबंधित प्रश्न
Copper sulphate solution is electrolysed using a platinum cathode and carbon anode.
Study the diagram given alongside and answer the following questions:
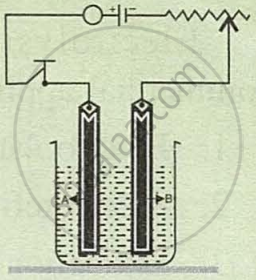
- Give the names of the electrodes A and B.
- Which electrode is the oxidising electrode?
During the electrolysis of copper sulphate solution, if ______ is used as electrodes, the colour of the electrolyte does not fade.
During electrolysis of acidulated water, the gas liberated at the anode is ______.
