Advertisements
Advertisements
प्रश्न
Why does microwave oven heats up a food item containing water molecules most efficiently?
उत्तर
The microwave oven heats up the food items containing water molecules most efficiently because the frequency of microwaves matches the resonant frequency of water molecules.
APPEARS IN
संबंधित प्रश्न
To which part of the electromagnetic spectrum does a wave of frequency 5 × 1019 Hz belong?
The wavelengths for the light of red and blue colours are roughly 7.8 × `10^7` m and 4.8 × `10^7` m respectively.
(a) Which colour has the greater speed in vacuum?
(b) Which colour has the greater speed in glass?
Give the range of wavelength of the electromagnetic waves visible to us.
Can a hydrogen atom emit characteristic X-rays?
X-ray incident on a material
(a) exerts a force on it
(b) transfers energy to it
(c) transfers momentum to it
(d) transfers impulse to it.
X-rays, gamma rays and microwaves travelling in a vacuum have ______.
An electron beam is accelerated by a potential difference V to hit a metallic target to produce X-rays. It produces continuous as well as characteristic X-rays. If λmin is the smallest possible wavelength of X-ray in the spectrum, the variation of log λmin with log V is correctly represented in:
The fundamental frequency of an open organ pipe is 300 Hz. The first overtone of this pipe has same frequency as first overtone of a closed organ pipe. If speed of sound is 330 m/s, then the length of closed organ pipe is:
What is the wavelength range of electromagnetic radiation used in radio broadcast?
In an atom X, electrons absorb the energy from an external source. This energy “excites” the electrons from a lower-energy level to a higher-energy level around the nucleus of the atom. When electrons return to the ground state, they emit photons.
The figure below is the energy level diagram of atom X with three energy levels, E1 = 0.00eV, E2 = 1.78eV and E3 = 2.95eV. The ground state is considered 0 eV for reference. The transition of electrons takes place between levels E1 and E2.
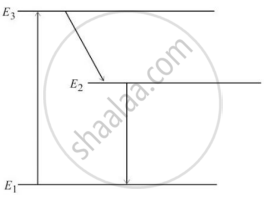
- What wavelength of radiation is needed to excite the atom to energy level E2 from E1?
- Suppose the external source has a power of 100 W. What would be the rate of photon emission?
