Advertisements
Advertisements
प्रश्न
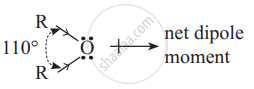
Why ethers possess a small net dipole moment?
टीपा लिहा
उत्तर
Since \[\begin{array}{cc}
|\phantom{.........}|\\
\ce{- C - O - C -}\\
|\phantom{.........}|
\end{array}\]bond angle is 110° and not 180°, the bond dipole moments of the two C - O bonds do not cancel each other; therefore ethers possess a small net dipole moment.
shaalaa.com
Ethers
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
