Advertisements
Advertisements
प्रश्न
Write the name and general formula of a chain of hydrocarbons in which an addition reaction with hydrogen is possible. State the essential condition for an addition reaction. Stating this condition, write a chemical equation giving the name of the reactant and the product of the reaction.
Write the name and general formula of a chain of hydrocarbons in which an addition reaction with hydrogen can take place. Stating the essential conditions required for an addition reaction to occur, write the chemical equation giving the name of the reactant and the product of such a reaction.
उत्तर १
Name and general formula of hydrocarbons undergoing addition reaction with hydrogen:
| Name | General Formula |
| Alkene | CnH2n |
| Alkyne | CnH2n-2 |
Essential conditions required for the addition reaction to occur:-
- Multiple bonds (double and triple bonds) must be present between carbon atoms in the chain of hydrocarbon.
- Addition of hydrogen should be carried out in the presence of catalyst such as nickel or platinum.
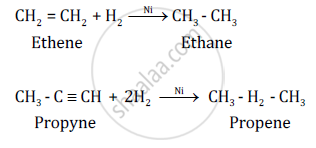
Chemical Equation:-

उत्तर २
The addition of hydrogen is possible in alkenes and alkynes. This is because of the presence of double and triple bonds, respectively. The general formula of alkenes is CnH2n and that of alkynes is CnH2n−2.
Conditions for addition reactions are
- Presence of an unsaturated compound, i.e. an unsaturated hydrocarbon.
- Presence of a species to be added to an unsaturated compound.
- Presence of a catalyst such as finely divided palladium or nickel.
APPEARS IN
संबंधित प्रश्न
Select saturated hydrocarbons from the following: C3H6; C5H10; C4H10; C6H14; C2H4
Why does the element carbon from a large number of carbon compounds?
What is the unique property of carbon atom? How is this property helpful to us?
An alkyne has seventy five carbon atoms in its molecule. The number of hydrogen atoms in its molecule will be:
(a) 150
(b) 148
(c) 152
(d) 146
The pair of elements which exhibits the property of catenation is:
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
if B is an open chain compound
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
Which compound contains single bonds as well as a double bond?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which formula represent cyclohexane as well as hexene?
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
Draw the structure of propanone.
