Advertisements
Advertisements
प्रश्न
Write the equation for the following:
Oxidation of chloroform by air and light.
उत्तर
In the presence of light, chloroform is slowly oxidised by air to carbonyl chloride, also known as phosgene.
\[\ce{\underset{Chloroform}{CHCl3} + 1/2O2 ->[Light] \underset{Phosgene}{COCl2} + HCl}\]
APPEARS IN
संबंधित प्रश्न
How the following conversion can be carried out?
Isopropyl alcohol to iodoform
Write balanced chemical equation for the following reaction:
Methyl magnesium iodide is treated with carbon dioxide and the product hydrolysed in acidic medium.
Chloroform and conc. HNO3 reacts to produce ____________.
Major product obtained on reaction of 3-Phenyl propene with HBr in presence of organic peroxide.
How can you obtain iodoethane from ethanol when no other iodine-containing reagent except NaI is available in the laboratory?
The most stable conformation of 1, 2-dibromomethane among the following is
 |
| I |
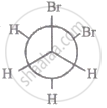 |
| II |
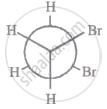 |
| III |
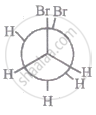 |
| IV |
Westrosol is:-
The compound that will not give iodoform on treatment with alkali and iodine is ______.
Which among the following pairs has only herbicides?
Write two uses of polyhalogen compounds.
