Advertisements
Advertisements
Question
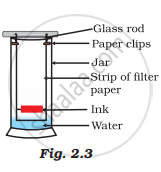
A child wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in Fig.2.3. The filter paper was removed when the water moved near the top of the filter paper.
(i) What would you expect to see, if the ink contains three different coloured components?
(ii) Name the technique used by the child.
(iii) Suggest one more application of this technique.
Solution
(i) The components of the ink will travel with water and we would see three bands on the filter paper at various lengths.
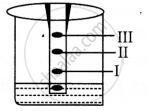
(ii) The technique is called chromatography.
(iii) Separation of pigments present in chlorophyll.
APPEARS IN
RELATED QUESTIONS
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Calculate the amount of carbon dioxide that could be produced when 1 mole of carbon is burnt in 16 g of dioxygen.
Explain the term molality
Solve the following problem:
Write the following number in ordinary decimal form:
3.49 × 10−11
Solve the following problem:
Write the following number in ordinary decimal form:
5.16 × 104
Solve the following problem:
Perform the following calculation. Round off your answer to two digits.
`1/(3.40xx10^24)`
Solve the following problem:
A 1.000 mL sample of acetone, a common solvent used as a paint remover, was placed in a small bottle whose mass was known to be 38.0015 g.
The following values were obtained when the acetone - filled bottle was weighed: 38.7798 g, 38.7795 g and 38.7801 g. How would you characterise the precision and accuracy of these measurements if the actual mass of the acetone was 0.7791 g?
Solve the following problem:
Your laboratory partner was given the task of measuring the length of a box (approx 5 in) as accurately as possible, using a metre stick graduated in milimeters. He supplied you with the following measurements:
12.65 cm, 12.6 cm, 12.65 cm, 12.655 cm, 126.55 mm, 12 cm.
Give your reason for rejecting each of the others.
Sulphuric acid reacts with sodium hydroxide as follows:
\[\ce{H2SO4 + 2NaOH -> Na2SO4 + 2H2O}\]
When 1 L of 0.1 M sulphuric acid solution is allowed to react with 1 L of 0.1 M sodium hydroxide solution, the amount of sodium sulphate formed and its molarity in the solution obtained is:
(i) 0.1 mol L–1
(ii) 7.10 g
(iii) 0.025 mol L–1
(iv) 3.55 g
The molarity of pure water is ______.
