Advertisements
Advertisements
Question
A graph of volume of hydrogen released vs time for the reaction between zinc and dil.HCl is given in figure. On the basis of this mark the correct option.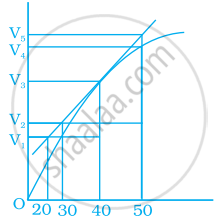
Options
Average rate upto 40s is `(V_3 - V_2)/40`
Average rate upto 40 seconds is `(V_3 - V_2)/(40 - 30)`
Average rate upto 40 seconds is `V_3/40`
Average rate upto 40 seconds is `(V_3 - V_1)/(40 - 20)`
MCQ
Solution
Average rate upto 40 seconds is `V_3/40`
Explanation:
Average rate for the reaction = `("Change in concentration of" H_2)/"Change in time"`
= `"Final concentration - Initial concenrtation"/"Final time - Initial time"`
Analyzing the graph line where time and vloume intersect.
= `(V_3 - 0)/(40 - 0)`
= `V_3/40`
shaalaa.com
Is there an error in this question or solution?
