Advertisements
Advertisements
Question
- Phenyl methenamine
- N, N - Dimethylaniline
- N - Methyl aniline
- Benzenamine
Choose the correct order of the basic nature of the above amines.
Options
A > C > B > D
D > B > C > A
D > C > B > A
A > B > C > D
MCQ
Solution
A > B > C > D
Explanation:
| (A) Phenyl methenamine : pKb = 4.7 | 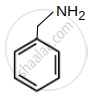 |
| (B) N, N - Dimethylaniline : pKb = 8.92 | 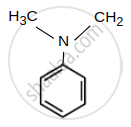 |
| (C) N - Methyl aniline : pKb = 9.3 | 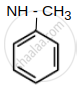 |
| (D) Benzenamine : pKb = 9.38 | 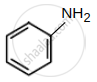 |
(A)> (B) > (C) > (D)
Alkyl groups donate electrons, increasing the amine's basicity. Because of the delocalization of the lone pair on nitrogen in the ring, aniline has the least fundamental property.
shaalaa.com
Is there an error in this question or solution?
