Advertisements
Advertisements
Question
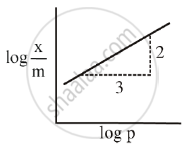
Adsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The x plot of log `x/m` versus log p is shown in the given graph. `x/m` is proportional to ______.
Options
`p^(2//3)`
`p^(3//2)`
p3
p2
Solution
Adsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The x plot of log `x/m` versus log p is shown in the given graph. `x/m` is proportional to `underline(p^(2//3))`.
Explanation:
According to Freundlich adsorption isotherm
`x/m = "Kp"^(1//"n")`
Taking logarithm on both sides we get
`log x/m = log "K" + 1/"n" log "p"`
Comparing equation for straight line we get,
y = mx + C
Slope (m) = `1/"n"`
`1/"n"` value lies between 0 and 1
It is only possible when `1/"n" = 2/3`
