Advertisements
Advertisements
Question
An alkane C8H18 is obtained as the only product on subjecting a primary alkyl halide to Wurtz reaction. On monobromination this alkane yields a single isomer of a tertiary bromide. Write the structure of alkane and the tertiary bromide.
Solution
The reaction is
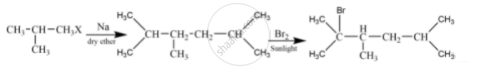
The alkane is
\[\begin{array}{cc}
\phantom{}\ce{H3C}\phantom{.........................}\ce{CH3}\phantom{}\\
\phantom{}\backslash\phantom{.......................}/\phantom{}\\
\phantom{}\ce{CH - CH2 - CH2 - CH}\phantom{}\\
\phantom{....}/\phantom{.......}|\phantom{................}\backslash\phantom{....}\\
\phantom{...}\ce{H3C}\phantom{........}\ce{CH3}\phantom{..............}\ce{CH3}\phantom{...}
\end{array}\]
The tertiary bromide is
\[\begin{array}{cc}
\phantom{..}\ce{H3C}\phantom{..}\ce{Br}\phantom{..................}\ce{CH3}\phantom{..}\\
\phantom{}\backslash\phantom{.}|\phantom{..................}/\phantom{}\\
\phantom{}\ce{C - \overset{H}{C} - CH2 - CH}\phantom{}\\
\phantom{....}/\phantom{.....}|\phantom{..............}\backslash\phantom{....}\\
\phantom{....}\ce{H3C}\phantom{.....}\ce{CH3}\phantom{.............}\ce{CH3}\phantom{...}
\end{array}\]
