Advertisements
Advertisements
Question
An atom consists of charged particles called electrons and protons. Each proton has a charge of +1 and each electron has a charge of –1. Remember number of electrons is equal to number of protons, while answering these questions:
What will be the charge on an atom if it loses an electron?
Solution
If an atom loses an electron, it will have (a – 1) electrons and a protons.
∴ Charge in one electron = (–1)
∵ Charge in (a-1) electrons = (a – 1) × (–1) = – (a – 1) = (1 – a)
∴ Charge in one proton = (+1)
∵ Charge in a protons = (+1) × a = (+a)
Hence, total charge on the atom = Charge of electrons + Charge of protons
= 1 – a + a
= + 1
APPEARS IN
RELATED QUESTIONS
Following number line shows the temperature in degree celsius (°C) at different places on a particular day
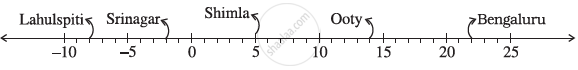
Observe this number line and write the temperature of the places marked on it.
Use the sign of >, < or = in the box to make the statements true.
(– 3) + 7 – (19) ![]() 15 – 8 + (– 9)
15 – 8 + (– 9)
Write the opposite of the following: Going 6m to the east
Find the sum of: −3057 and 199
Find the sum of: −18, + 25 and −37
Find an integer a such that: a + 6 = 0
Multiply: 18 by −7
Find the product: (−3) × (−7) × (−6)
−5 − (−8) = ?
Evaluate: (–40) ÷ (–10)
