Advertisements
Advertisements
Question
An ideal gas is expanded isothermally from volume V1 to volume V2 and then compressed adiabatically to original volume V1. If the initial pressure is P1, the final pressure is P3 and net work done is W, then ____________.
Options
P3 >P1, W > 0
P3 < P1, W < 0
P3 > P1, W < 0
P3 = P1, W = 0
Solution
An ideal gas is expanded isothermally from volume V1 to volume V2 and then compressed adiabatically to original volume V1. If the initial pressure is P1, the final pressure is P3 and net work done is W, then P3 > P1, W < 0.
Explanation:
Figure shows the PV diagram of the given process.
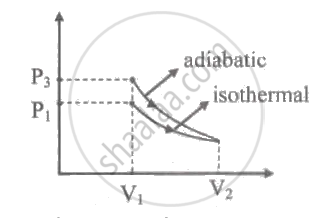
From figure it can be said that, area under the curve for isothermal expansion is lesser than area under the curve for adiabatic compression.
∴ The work done is negative. Also, pressure P3 > P1
