Advertisements
Advertisements
Question
Arrange the following as per the instruction given in the brackets:
Na, K, Li (Increasing atomic size)
Solution
Li < Na < K
APPEARS IN
RELATED QUESTIONS
Arrange the following as per the instruction given in the bracket:
Mg, Cl, Na, S, Si (decreasing order of atomic size).
What do you understand by atomic size? State its unit?
Give the trends in atomic size on moving down the group.
Arrange the following in order of increasing radii:
CI- , CI
On moving from left to right in a periodic table, the size of the atom _______.
An element X has mass number 40 and contains 21 neutrons in its atom. To which group of the Periodic Table does it belong?
- Electropositive nature of the element(s) increases down the group and decreases across the period
- Electronegativity of the element decreases down the group and increases across the period
- Atomic size increases down the group and decreases across a period (left to right)
- Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
- Name the most electropositive element among them
- Name the most electronegative element
- Name the element with smallest atomic size
- Name the element which is a metalloid
- Name the element which shows maximum valency.
The diagram below shows part of the periodic table.
- Which elements would react together to form covalent compounds?
- Between the two elements W and Z, which will have a bigger atomic radius? Why?
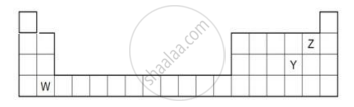
Which one has the largest size?
This question refers to the elements of the Periodic Table with atomic numbers from 3 to 18. Some of the elements are shown by letters, but the letters are not the usual symbols of the elements.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | B | C | D | E | F | G | H |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| I | J | K | L | M | N | O | P |
Which of these have least atomic size in period 3?
