Advertisements
Advertisements
Question
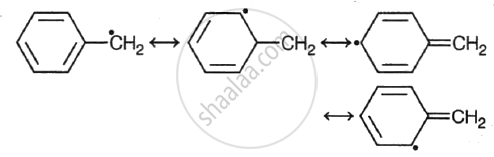
Arrange the following free radicals in order of decreasing stability.
- Methyl
- Vinyl
- Allyl
- Benzyl
Options
I > II > III > IV
III > II > I > IV
II > I > IV > III
IV > III > I > II
MCQ
Solution
IV > III > I > II
Explanation:
Among the alkyl free radicals, tertiary alkyl free radicals are most stable and methyl free radical is least stable.
Alkyl free radicals are less stable than benzyl and allyl free radicals, which are resonance stabilised. Due to greater conjugation, the free radical of benzoyl is more stable than that of allyl. Since the sp3 C-atom has an extra electron, the vinyl radical is the least stable.
shaalaa.com
Theoretical Basis of Organic Reactions
Is there an error in this question or solution?
