Advertisements
Advertisements
Question
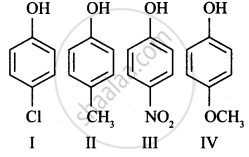
Arrange the following in decreasing order of acidic character:
Options
IV > III > I > II
II > IV > I > III
I > II > III > IV
III > I > II > IV
MCQ
Solution
III > I > II > IV
Explanation:
Since –NO2 and Cl are electron-withdrawing but NO2 > Cl.
–OCH3 is more electron releasing due to the +R effect than –CH3 group, therefore, IV is the least acidic.
shaalaa.com
Is there an error in this question or solution?
