Advertisements
Advertisements
Question
Attempt the following:
What is meant by LDP and HDP? Mention the basic difference between the same with suitable examples.
Solution
LDP is a branched polymer of ethene with polymeric chains loosely held. Hence, even though it is tough, it is extremely flexible. Therefore, LDP is used in producing extruded films, sheets, mainly for packaging and household uses like in preparation of squeeze bottles, attractive containers, etc. where low tensile strength and flexibility are required.
On the other hand, HDP is a linear polymer of ethene with closely packed polymeric chains. Hence, it is much stiffer than LDP and has high tensile strength and hardness. Therefore, HDP is used in the manufacture of toys and other household articles like buckets, dustbins, bottles, pipes, laboratory wares, and other objects where high tensile strength and stiffness are required.
APPEARS IN
RELATED QUESTIONS
Bakelite is the polymer of:
(a) Benzaldchyde and phenol
(b) Acetaldehyde and phenol
(c) Formaldehyde and phenol
(d) Formaldehyde and benzyl alcohol
Write the structures of the monomers used for getting the following polymers
Melamine – formaldehyde polymer
Answer the following in one sentence.
Identify 'A' in the following reaction:
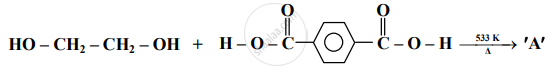
Answer the following in one sentence.
Identify thermoplastic and thermosetting plastic from the following:
- PET
- Urea formaldehyde resin
- Polythene
- Phenol formaldehyde resin
Answer the following.
Match the following pairs:
| Name of polymer | Monomer |
| 1. Teflon | a. CH2 = CH2 |
| 2. PVC | b. CF2 = CF2 |
| 3. Polyester | c. CH2 = CHCl |
| 4. Polythene | d. C6H5OH and HCHO |
| 5. Bakelite | e. Dicarboxylic acid and polyhydoxyglycol |
Draw the structures of polymers formed from the following monomers
\[\ce{n HOOC–R–COOH + n HO–R'–OH}\]
Name and draw structure of the repeating unit in natural rubber.
Answer the following.
Write name and formula of raw material from which bakelite is made.
Attempt the following:
Explain the vulcanisation of rubber. Which vulcanizing agents are used for the following synthetic rubber?
a. Neoprene
b. Buna-N
Answer the following.
Is synthetic rubber better than natural rubber? If so, in what respect?
Write the name of the catalyst used for preparation of high density polythene polymer.
Monomers ethylene glycol and terephthalic acid undergo condensation polymerization to give polymer called ___________
Write a chemical reaction for the preparation of the following polymer.
polyacrylonitrile
Write chemical reaction for preparation of the following.
Neoprene
Write chemical reactions for the preparation of high-density polythene.
Write two uses and two properties of polythene.
Explain the reactions involved in the preparation of viscose rayon.
The following structure represents the polymer:
\[\begin{array}{cc}
\ce{[-C-CH2-NH-C-(-CH2)5 NH -]_{{n}}}\\
\phantom{}||\phantom{.............}||\phantom{................}\\
\phantom{}\ce{O}\phantom{.............}\ce{O}\phantom{................}
\end{array}\]
Which among the following polymers is obtained from styrene and 1-3-butadiene?
Which among the following polymers is used for making handles of cooker?
Identify additional polymers from the following.
I. \[\begin{array}{cc}
\ce{-(CH2 - CH -)_{{n}}}\\
\phantom{....}|\\
\phantom{.......}\ce{C6H5}
\end{array}\]
II. \[\ce{-(CH2 - CH = CH - CH2 -)_{{n}}}\]
III. \[\ce{-(CO(CH2)4 - CONH(CH2)6NH -)_{{n}}}\]
IV.
![]()
How many isoprene units are present in abscisic acid?
Identify the CORRECT statement regarding the following polymer.
\[\begin{array}{cc}
\phantom{....}\ce{O}\phantom{............}\ce{O}\phantom{...................}\ce{H}\phantom{.....}\\
\phantom{....}||\phantom{.............}||\phantom{...................}|\phantom{......}\\
\ce{-[C - (CH2)4 - C - NH - (CH2)6 - N -]_{{n}}}
\end{array}\]
Which of the following is the monomer of neoprene?
\[\ce{{n} CH2 = CH2 ->[333 K - 343 K][6 - 7 atm, catalyst] X}\]
Which of the following is CORRECT about polymer 'X'?
Which of the following compounds is used to prepare orlon?
Which among the following catalysts is used in the preparation of dacron?
Which of the following monomers is used in the manufacture of Neoprene rubber?
Which of the following polymers is used as insulation for cables?
Which of the following is not a semisynthetic polymer?
Which of the following polymers, need atleast one diene monomer for their preparation?
(i) Dacron
(ii) Buna-S
(iii) Neoprene
(iv) Novolac
Match the polymers given in Column I with the type of linkage present in them given in Column II.
| Column I | Column II |
| (i) Terylene | (a) Glycosidic linkage |
| (ii) Nylon | (b) Ester linkage |
| (iii) Cellulose | (c) Phosphodiester linkage |
| (iv) Protein | (d) Amide linkage |
| v) RNA |
Which one of the following polymers are prepared by addition polymerization?
Which of the following is an example of polyester?
Which of the following polymers is synthesized using a free radical polymerisation technique?
Nylon threads are made of ______.
Polymer used in bullet proof glass is ______.
Identify the monomer used to prepare neoprene.
Name and draw the structure of the repeating unit in natural rubber.
Name and draw the structure of the repeating unit in natural rubber.
How the Bakelite is prepared? Give the steps involved in the preparation.
Write the structure and name of monomer of Natural rubber.
Name and draw the structure of the repeating unit in natural rubber.
Name and draw the structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
