Advertisements
Advertisements
Question
Calcium oxide reacts vigorously with water to produce slaked lime.
This reaction can be classified as:
(A) Combination reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
Options
(A) and (C)
(C) and (D)
(A), (C) and (D)
(A) and (B)
Solution
When calcium oxide reacts with water it produces slaked lime and a huge amount of heat is released, this reaction can be classified as a combination reaction.
Hence, the correct answer is (A) and (B).
RELATED QUESTIONS
Gas A, which is the major cause of global warming, combines with hydrogen oxide B in nature in the presence of an environmental factor C and a green material D to form a six carbon organic compounds E and a gas F. The gas F is necessary for breathing.
(a) What is gas A?
(b) What is the common name of B?
(c) What do you think could be C?
(d) What is material D? Where is it found?
(e) Name the organic compound E.
(f) What is gas F? Name the natural process during which it is released.
What type of reaction is represented by the following equation?
CaO + H2O → Ca(OH)
Define a chemical reaction.
Fill in the blank
A reaction in which two or more substances combine to form a single substance is called a ............ reaction.
Select the correct answer for the statement given below:
The product formed during direct combination reaction of carbon dioxide and water.
Complete the statement by filling in the blank with the correct word:
Direct combination reaction of phosphorus pentoxide with water gives _______.
H2(g) + Cl29(g) → 2HCl(g) is a ______
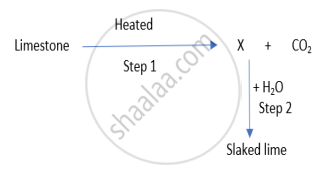
Identify the correct option from the given table which represents the type of reactions occurring in step 1 and step 2.
Balance the following chemical equation and identify the type of chemical reaction.
Read the text below and answer the questions that follow:
A small amount of hydrochloric acid was taken in a test tube. The test tube was heated. A glass rod was dipped in the ammonia solution and held on the top of the test tube. A white smoke was seen emanating from the tip of the glass rod.
- What must have happened?
- Which colour of gas is formed?
- Write the chemical equation for the reaction.
