Advertisements
Advertisements
Question
Calculate the number of neutrons present in the nucleus of an element X which is represented as `""_15^31"X"`
Solution
""_15^31"X"` number of protons = number of electrons = 15
Number of neutrons =Mass number – no. of protons
= 31 – 15
= 16
APPEARS IN
RELATED QUESTIONS
Compare the properties of electrons, protons and neutrons.
A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
The mass of an electron is about `1/2000` times that of proton.
Name the element which does not contain any neutrons in its nucleus.
Explain in brief the experimental proof which led to the discovery of –
Neutrons
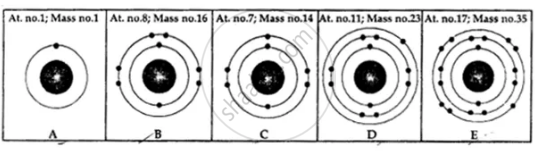
State the number of neutrons in each of the atoms A to E. Also state which of the atoms A to E is a metal.
James Chadwick discovered the fundamental particle called proton.
Write the properties of neutrons.
α, β, γ rays are during the ratio active decay ______ of an atom.
The ratio of neutrons in C and Si with respective atomic masses 12 and 28 is ______.
