Advertisements
Advertisements
Question
Calculate the relative molecular mass of:
Potassium chlorate
Solution
KClO3 = (K)39 + (Cl)35.5 + (3O)48 = 122.5
APPEARS IN
RELATED QUESTIONS
Give balanced chemical equations for the following conversions A, B, and C:
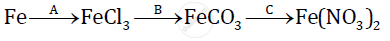
Aluminium carbide reacts with water according to the following equation :
`Al_4C_3 + 12H_2O-> 4Al(OH)_3 + 3CH_4`
1)What mass of aluminium hydroxide is formed from 12g of aluminium carbide?
2) What volume of methane at s.t.p. is obtained from 12g of aluminium carbide?
[Relatively molecular weight of `Al_4Cl_3 = 144; Al(OH)_3 = 78]`
What weight of sulphuric acid will be required to dissolve 3g of magnesium carbonate?
[Mg = 24, C =12, 0 = 16 ]
MgCO3 + H2SO4 → MgSO4 + H2O+ CO2
Concentrated nitric acid oxidizes phosphorous to phosphoric acid according to the following equation :
P + 5HNO3 → H3PO4 + H2O + 5NO2
What mass of phosphoric acid can be prepared from 6 .2 g of phosphorous?
Washing soda has the formula Na2CO3.10H2O.What is the mass of anhydrous sodium carbonate left when all the water of crystallization is expelled by heating 57.2 g of washing soda?
When heated, potassium permanganate decomposes according to the following equation:
\[\ce{2KMnO4 -> \underset{solid residue}{K2MnO4 + MnO2} + O2}\]
Given that the molecular mass of potassium permanganate is 158 g, what volume of oxygen (measured at room temperature) would be obtained by the complete decomposition of 15.8 g of potassium permanganate? (Molar volume at room temperature is 24 litres). [K = 39, Mn = 55, O = 16]
The equation for the burning of octane is:
\[\ce{2C6H18 + 25O2 -> 16CO2 + 18H2O}\]
If the relative molecular mass of carbon dioxide is 44, what is the mass of carbon dioxide produced by burning two moles of octane?
Calculate the number of atoms of each kind in 5.3 grams of sodium carbonate.
Calculate the mass of nitrogen supplied to soil by 5 kg of urea [CO(NH2)2].
[O = 16; N = 14; C = 12; H = 1]
What do you understand by the statement that 'vapour density of carbon dioxide is 22'?
