Advertisements
Advertisements
Question
Define: Atomic number
Solution
Atomic number— Atomic number refers to the number of protons present in an atom. It is denoted by Z.
APPEARS IN
RELATED QUESTIONS
Write down the name of a compound represented by the following formula:
K2SO4
Write down the name of a compound represented by the following formula:
CaCl2
Write down the name of a compound represented by the following formula:
CaCO3
State the term.
The number of atoms present in a molecule of an element _________
Define: Atomic number
Define: Mass number :
What is the atomicity of the following ?
- Oxygen
- Ozone
- Neon
- Sulphur
- Phosphorus
- Sodium
Work out the formula for magnesium hydrogencarbonate.
An element A forms an oxide A2O5.
What is the valency of element A ?
An element X forms the following compounds with hydrogen, carbon and oxygen :
H2X, CX2, XO2, XO3
State the three valencies of element X which are illustrated by these compounds.
Correct the following statement:
A molecule of sulphur is monoatomic.
Explain the term – molecules. Give three examples of atoms of the same element forming a molecule. State the atomicity of the same.
State the main points of the contradiction of Dalton’s atomic theory by the Modern Atomic Theory.
Give a reason why –
Helium is considered monoatomic, but hydrogen a diatomic molecule.
State the atomicity of the following:
Neon
State the atomicity of the following:
Chlorine
State the atomicity of the following:
Argon
State the atomicity of the following:
Sulphur
State true of false. If false, give the correct statement.
NaCl represents one molecule of sodium chloride.
Classify the following molecules as the molecules of element or compound
| 1. |  |
|
| 2. |  |
|
| 3. | 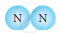 |
|
| 4. | 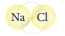 |
If a molecule is made of similar kinds of atoms, then it is called ______ atomic molecule.
Give any two examples for heterodiatomic molecules.
