Advertisements
Advertisements
Question
Describe an experiment to show that water has maximum density at 4°C. What important consequences follow this peculiar property of water? Discuss the importance of this phenomenon in nature.
Solution
Hope's experiment to demonstrate that water has maximum density at 4°C :

Hope's apparatus consists of a tall metallic cylinder provided with two side openings P and Q, P near the top and Q near the bottom, fitted with thermometers T1 and T2 in them. The central part of the cylinder is surrounded with a cylindrical trough containing a freezing mixture of ice and salt. The cylinder is fitted with pure water at room temperature.
Observations: (i) Initially, both thermometers T1 and T2 are at the same temperature.
(ii) First, the temperature recorded by the lower thermometer T2 starts decreasing and finally it becomes steady at 4°C, while the temperature recorded in the upper thermometer T1 remains almost unchanged during this time.
(iii) Then, the temperature recorded by the lower thermometer T2 remains constant at 4°C and upper thermometer T1 records a continuous fall in temperature up to 0°C and then it becomes steady.
Thus, finally, the temperature recorded by the upper thermometer is 0°C and that by the lower thermometer is 4°C.
As the freezing mixture cools water in the central portion of the cylinder, water contracts and its density increases, consequently it sinks to the bottom, thereby causing the reading of the lower thermometer T2 to fall rapidly. The reading of the upper thermometer T1 does not change as the temperature of water in the upper part does not change. This continues till the entire water below the central portion reaches 4°C. On cooling further below 4, due to anomalous expansion, the water of the central portion expands, so its density decreases and hence it rises up. As a result, reading of the upper thermometer T1 falls rapidly to 0°C and water freezes to form ice at 0°C near the top. This proves that water has a maximum density at 4°C.
This anomalous expansion of water helps in preserving the aquatic life during the very cold weather. In winters, when the temperature falls, the top layer of water in a pond contract, becomes denser and sinks to the bottom. A circulation is thus set up until the entire water in the pond reaches its maximum density at 4°C. If the temperature falls further, then the top layer expands and remains on the top till it freezes. Thus, even though the upper layers are frozen, the water near the bottom is at 4°C and the fishes can survive in it easily.
APPEARS IN
RELATED QUESTIONS
Give reason for Sea water does not freeze at 0°C.
What do you mean by the anomalous expansion of water?
Explain the following
Fishes survive in ponds even when the atmospheric temperature is well below 0°C.
Explain why do vegetables and fruits get damaged during severe frost?
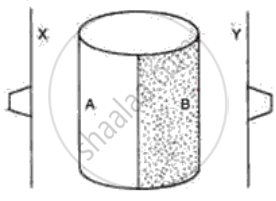
The following figure shows a metal cylinder, containing boiling water. One half side A is polished and another half, B is painted black. Two thin metal sheets X and Y are painted black and have one rubber stopper fixed with wax on each sheet. These sheets are equidistant from the boiling water (container A, B) as shown in the diagram. What would you expect to happen after a few minutes? Give a reason for your answer.
_______ apparatus is used to study the anomalous behaviour of water.
Draw a neat and labelled diagram of Hope’s apparatus.
