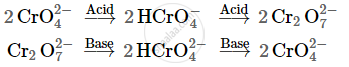Advertisements
Advertisements
Question
Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Solution
Potassium dichromate is prepared from chromite ore (FeCr2O4) in the following steps.
Step (1): Preparation of sodium chromate
`4FeCr_2O_4 + 16NaOH+7O_2 -> 8Na_2CrO_4+2Fe_2O_3+8H_2O`
Step (2): Conversion of sodium chromate into sodium dichromate
`2Na_2CrO_4 + conc.H_2SO_4 -> Na_2Cr_2O_7 + Na_2SO_4 + H_2O`
Step(3): Conversion of sodium dichromate to potassium dichromate
`Na_2Cr_2O_7 + 2KCl -> K_2Cr_2O_7 + 2NaCl`
Potassium dichromate being less soluble than sodium chloride is obtained in the form of orange coloured crystals and can be removed by filtration.
The dichromate ion `(Cr_2O_7^(2-))` exists in equilibrium with chromate `(CrO_4^(2-))` ion at pH 4. However, by changing the pH, they can be interconverted.