Advertisements
Advertisements
Question
Discuss how high specific heat capacity of water helps in formation of land and sea breeze.
Solution
Specific heat capacity of land is 5 times less as compared to water. Thus, air above land becomes hot and light and rises up resulting in drop in pressure of land mass during day time. Thus, cool air from sea starts blowing towards and forming sea breeze.
During night, land as well as sea radiates heat energy. However, temperature of land falls faster than sea water, because of high specific heat capacity of sea water. So, at night the temperature of sea water is more than land. Warm air above the sea rises and cold air from land starts blowing towards sea resulting in land breeze.
APPEARS IN
RELATED QUESTIONS
A mass m1 of a substance of specific heat capacity c1 at temperature t1 is mixed with a mass m2 of other substance of specific heat capacity c2 at a lower temperature t2. Deduce the expression for the temperature t of the mixture. State the assumption made, if any.
Explain the term boiling ?
Study the following procedure and answer the questions below:
1. Take 3 spheres of iron, copper and lead of equal mass.
2. Put all the 3 spheres in boiling water in a beaker for some time.
3. Take 3 spheres out of the water. Put them immediately on a thick slab of wax.
4. Note, the depth that each sphere goes into the wax.
i) Which property of substance can be studied with this procedure?
ii) Describe that property in minimum words.
iii) Explain the rule of heat exchange with this property.
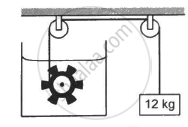
Figure shows a paddle wheel coupled to a mass of 12 kg through fixed frictionless pulleys. The paddle is immersed in a liquid of heat capacity 4200 J K−1 kept in an adiabatic container. Consider a time interval in which the 12 kg block falls slowly through 70 cm. (a) How much heat is given to the liquid? (b) How much work is done on the liquid? (c) Calculate the rise in the temperature of the liquid neglecting the heat capacity of the container and the paddle.
A liquid X has specific heat capacity higher than the liquid Y. Which liquid is useful as heat reservoir to keep juice bottles without freezing?
Numerical Problem.
How much heat energy is required to change 2 kg of ice at 0°C into water at 20°C? (Specific latent heat of fusion of water = 3,34,000 J/kg, Specific heat capacity of water = 4200 JKg–1K–1).
When a uniform rod is heated, which of the following quantity of the rod will increase
Two metals A and B have specific heat capacities in the ratio 2 : 3. If they are supplied the same amount of heat then
Which metal piece will show a greater rise in temperature given their masses is the same?
Why is water used as a coolant in radiators of a car?
