Advertisements
Advertisements
Question
Discuss SN2 mechanism of methyl bromide using aqueous KOH.
Answer in Brief
Solution
- Consider alkaline hydrolysis of methyl bromide (Bromomethane ), CH3Br with aqueous NaOH or KOH.
\[\ce{\underset{substrate}{\underset{Bromomethane}{CH3 - Br}} + \underset{Nucleophile}{OH-} -> \underset{Methanol}{CH3 - OH} + Br-}\] - Stereochemistry and Kinetics of the reaction (R.D.S.): There is only one step in this hydrolysis reaction, and that step determines the rate, or R.D.S. The rate of hydrolysis reaction depends on the concentration of CH3Br and OH- which are present in the R.D.S. of the reaction.
Rate = R = k [CH3Br] [OH− ], where k is rate constant of the reaction.
SN2 reaction: Since the rate of the reaction is dependent on the concentrations of the two reactive species methyl bromide and hydroxide ion the reaction between these two substances to generate methanol follows second order kinetics. This means that the reaction is bimolecular second order (2nd) SN stands for Nucleophilic Substitution Reaction. - Mechanism of the reaction:
- It is a single-step mechanism. The reaction takes place in the following steps :
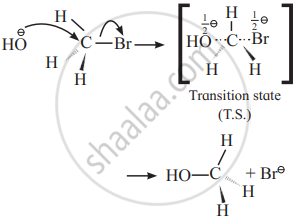
- Backside attack of the nucleophile: Nucleophile, OH− attacks carbon atom of CH3 Br from back side i.e., from opposite side to that of the leaving group, i.e. Br− to experience minimum steric repulsion and electrostatic repulsion between the incoming nucleophile (OH−) and leaving Br−.
- Transition state: When a nucleophile, OH− approaches carbon atom of CH3Br, the potential energy of the system increases until a transition state (T.S.) of maximum potential energy is formed in which C–Br bond is partially broken and C–OH bond is partially formed. The negative charge is equally shared by both incoming nucleophile –OH− and outgoing, leaving group –Br−. (Thus, the total negative charge is diffused.)
- In CH3Br, carbon atom is sp3 hybridized and CH3 Br molecule is tetrahedral. The hybridization of carbon atom changes to sp2 hybridization. The transition state contains carbon having three σ (sigma) bonds in one plane making bond angles of 120° with each other i.e., H1, H2 and H3 atoms lie in one plane while two partial covalent bonds containing Br and OH lie collinear and on opposite sides perpendicular to the plane.
- Inversion of configuration: The transition state decomposes fast by the complete breaking of the C–Br bond and the new C–OH bond is formed on the other side. The breaking of C–Br bond and the formation of C–OH bond take place simultaneously. The energy required to break the C–Br bond is partly obtained from the energy released when C–OH bond is formed. The formation of product CH3 OH is accompanied by complete or 100% inversion of configuration forming again sp3 hybridized carbon atom giving tetrahedral CH3 OH molecule. But in this structure the positions of H2 and H3 atoms in the reactant (CH3Br) and in product are on the opposite side. This inversion of configuration is called Walden inversion.
- It is a single-step mechanism. The reaction takes place in the following steps :
shaalaa.com
Is there an error in this question or solution?
