Advertisements
Advertisements
Question
Discuss the mechanism of alkaline hydrolysis of methyl bromide.
Answer in Brief
Solution
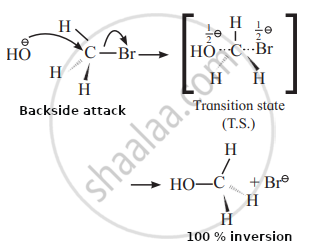
Alkaline hydrolysis of methyl bromide:
- It uses the SN2 mechanism, which is bimolecular nucleophilic substitution.
- \[\ce{CH3Br + KOH -> CH3OH + KBr}\]
- This is a one-step reaction in which bond formation and bond breaking at carbon occur simultaneously.
- To avoid steric repulsion or hindrance with the departing group, there is a backside attack of nucleophiles.
- This is a single-step reaction. As a result, there is only one transition state. The transition state has penta-coordinate carbon with three sigma bonds in one plane and the remaining two perpendicular to this plane.
- The backwise assault causes complete inversion, similar to flipping an umbrella.
Mechanism of Reaction:
shaalaa.com
Is there an error in this question or solution?
