Advertisements
Advertisements
Question
Draw the structural formula of a compound with two carbon atoms in the following case:
An alcohol containing two carbon atoms.
Solution
Ethanol
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{.....}\\
|\phantom{....}|\phantom{.....}\\
\ce{H - C - C - O - H}\\
|\phantom{....}|\phantom{.....}\\
\ce{H}\phantom{...}\ce{H}\phantom{.....}\\
\end{array}\]
APPEARS IN
RELATED QUESTIONS
In an experiment to study the properties of ethanoic acid, a student takes about 3 mL of ethanoic acid in a dry test tube. He adds an equal amount of distilled water to it and shakes the test tube well. After some time he is likely to observe that
(A) a colloid is formed in the test tube.
(B) the ethanoic acid dissolves readily in water.
(C) the solution becomes light orange.
(D) water floats over the surface of ethanoic acid.
A student puts a drop of reaction mixture of a saponification reaction first a blue litmus paper and then on a red litmus paper. He may observe that:
(a) There is no change in the blue litmus paper and the red litmus paper turns white.
(b) There is no change in the red litmus paper and the blue litmus paper turns red.
(c) There is no change in the blue litmus paper and the red litmus paper turns blue.
(d) No change in colour is observed in both the litmus papers
A student adds 2 mL of acetic acid to a test tube containing 2 mL of distilled water. He then shakes the test tube well and leaves it to settle for some time. After about 5 minutes he observes that in the test tube there is :
(A) a clear transparent colourless solution
(B) a clear transparent pink solution
(C) a precipitate settling at the bottom of the test tube
(D) a layer of water the layer of acetic acid
Give balanced chemical equations for Sodium ethanoate to methane.
Name one chemical compound which can be used to distinguish between ethanol and ethanoic acid.
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 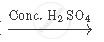
Explain the following term with an example.
Oxidant
What is vinegar and glacial acetic acid?
Choose the correct word/phrase from the options given below to complete the following sentence:
When acetaldehyde is oxidized with acidified potassium dichromate, it forms ______.
Bubbles are seen in the test tube during the preparation of lime water.
