Advertisements
Advertisements
Question
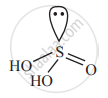
Draw the structure of oxyacid of sulphur in which the oxidation state of sulphur is + 4.
Short Note
Solution
Sulphurous acid, H2SO3 is an oxoacid of sulphur in which oxidation state of S is +4 (+ 2 + x - 6 = 0 ∴ x = +4)
Structure of H2SO3:
shaalaa.com
Oxoacids
Is there an error in this question or solution?
