Advertisements
Advertisements
Question
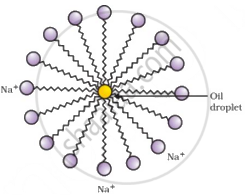
Elaborate on the process of cleansing action. Illustrate micelle with the help of labelled diagram.
Answer in Brief
Diagram
Solution
- Soaps are molecules in which the two ends have differing properties; one is hydrophilic, that is, it dissolves in water, while the other is hydrophobic, that is, it dissolves in hydrocarbons.
- The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic end of soap dissolves in water, while the carbon chain dissolves in oil. Thus, the soap molecules form micelles, where one end of the molecules is towards the oil droplet while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps dissolve the dirt in water, and we can wash our clothes clean.
shaalaa.com
Is there an error in this question or solution?
2024-2025 (March) Board Sample Paper
