Advertisements
Advertisements
Question
Explain how can a gas be expanded at constant temperature.
Answer in Brief
Solution
- Consider a thermodynamic system consisting of an ideal gas confined to a cylinder with a movable, frictionless, and massless piston.
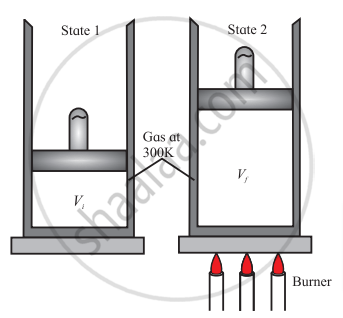
- When the gas is heated slowly, in a controlled manner so that it expands at a constant temperature. It reaches the final volume Vf isothermally.
- The system absorbs a finite amount of heat during this process.
- But as the process is very slow, the temperature remains constant.
- In this way, the gas expands and reaches final volume Vf at constant temperature.
shaalaa.com
Thermodynamic Process
Is there an error in this question or solution?
