Advertisements
Advertisements
Question
Explain Hydrogen Oxygen Fuel Cell with a neat diagram.
Solution
Definition of Fuel Cell: A fuel cell is a device that produces electricity through a chemical reaction between a source fuel and an oxidant.
In a fuel cell, the catalyst facilitates the reaction of oxygen and hydrogen. It is usually made of platinum powder very thinly coated onto carbon paper or cloth. The catalyst is rough and porous so the maximum surface area of the platinum can be exposed to the hydrogen or oxygen.
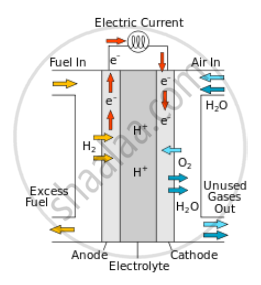
The Fuel cell consists an anode, a cathode and an electrolyte. Hydrogen is passed through the anode and oxygen is passed through cathode.
At the anode, hydrogen molecule gets split into electrons and protons. The protons pass through electrolyte and electrons generate electricity through circuit.
At cathode electrons, protons and oxygen combine to form a water molecule.
Chemical Reaction
Anode: \[\ce{H2->2H+ + 2e-}\]
Cathode: \[\ce{1/2O2 + 2H+ +2e- ->H2O}\]
Cell: \[\ce{H2 + 1/2O2 -> H2O}\]
Diagram: