Advertisements
Advertisements
Question
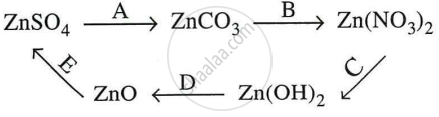
Give equations for the following conversions A to E.
Solution
A : \[\ce{\underset{zinc sulphate}{ZnSO4} + \underset{sodium carbonate}{Na2CO3}-> \underset{zinc carbonate}{ZnCO3} + \underset{sodium sulphate}{Na2SO4}}\]
B : \[\ce{\underset{zinc carbonate}{ZnCO3} + \underset{nitric acid}{2HNO3}-> \underset{zinc nitrate}{Zn(NO3)2} + \underset{water}{H2O} + \underset{carbon dioxide}{CO2\uparrow}}\]
C : \[\ce{\underset{zinc nitrate}{Zn(NO3)2} + \underset{sodium hydroxide}{2NaOH}-> \underset{zinc hydroxide}{Zn(OH)2\downarrow} + \underset{sodium nitrate}{2NaNO3}}\]
D : \[\ce{\underset{zinc hydroxide}{Zn(OH)2}->[\Delta]\underset{zinc oxide}{ZnO} + \underset{water}{H2O}}\]
E : \[\ce{\underset{zinc oxide}{ZnO} + \underset{sulphuric acid}{H2SO4}->\underset{zinc sulphate}{ZnSO4} + \underset{water}{H2O}}\]
