Advertisements
Advertisements
Question
Give four examples of heterogeneous catalysis.
Solution 1
In heterogeneous catalysis, the catalyst is present in a different phase than that of the reactants,e.g.,

Solution 2
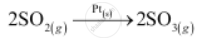
1) Oxidation of sulphur dioxide to form sulphur trioxide. In this reaction, Pt acts as a catalyst.
2)Formation of ammonia by the combination of dinitrogen and dihydrogen in the presence of finely divided iron.

This process is called the Haber’s process.
3) Oswald’s process: Oxidation of ammonia to nitric oxide in the presence of platinum.

4)Hydrogenation of vegetable oils in the presence of Ni.

APPEARS IN
RELATED QUESTIONS
What role does adsorption play in heterogeneous catalysis?
Give four uses of adsorption.
Which among the following ore is concentrated by froth floatation process?
Which of the following is an example of hydrophobic sol?
At the equilibrium position in the process of adsorption ______.
What are the applications of adsorption in chemical analysis?
Adsorption process is used in ______
