Advertisements
Advertisements
Question
Give the structural formulae of acetic acid.
Solution
\[\begin{array}{cc}
\ce{CH3 - C - OH}\\
\phantom{.}||\\
\phantom{.}\ce{O}\\
\end{array}\]
APPEARS IN
RELATED QUESTIONS
In order to study saponification reaction, we first prepare 20% solution of sodium hydroxide. If we record the temperature of this solution just after adding sodium hydroxide flakes to water and also test its nature using litmus, it may be concluded that the process of making this solution is
(A) exothermic and the solution is alkaline
(B) endothermic and the solution is alkaline
(C) endothermic and the solution is acidic
(D) exothermic and the solution is acidic
Write the IUPAC names, common names and formulae of the first two members of the homologous series of carboxylic acids.
Fill in the following blank with suitable word:
The next higher homologue of ethanol is ...............
Fill in the following blank with a suitable word:
The organic acid present in vinegar is ______.
What do you notice when acetic acid reacts with alkalies?
A student puts a drop of acetic acid first on a blue litmus paper and then on a red litmus paper. He would observe that
(A) the red litmus paper turns colourless and there is no change in the blue litmus paper.
(B) the red litmus paper turns blue and the blue litmus paper turns red.
(C) there is no change in the red litmus paper and the blue litmus paper turns red.
(D) there is no change in the blue litmus paper and the red litmus paper turns blue.
Write three physical properties of acetic acid.
Choose the correct word/phrase from the options given below to complete the following sentence:
When acetaldehyde is oxidized with acidified potassium dichromate, it forms ______.
Give balanced chemical equations for the following conversion:
Calcium carbide to ethyne
The reaction between acetic acid and sodium carbonate is shown in the following figure.
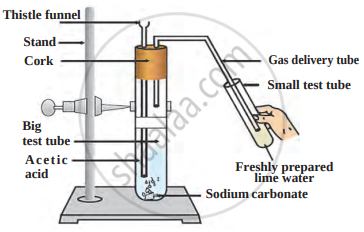
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
