Advertisements
Advertisements
Question
Given below are two statements:
Statement I: The esterification of carboxylic acid with an alcohol is a nucleophilic acyl substitution.
Statement II: Electron withdrawing groups in the carboxylic acid will increase the rate of esterification reactions.
Choose the most appropriate options.
Options
Both Statements I and Statement II are correct.
Both Statements I and Statement II are incorrect.
Statement I is correct but Statement II is incorrect.
Statement I is incorrect but Statement II is correct.
Solution
Both Statements I and Statement II are correct.
Explanation:
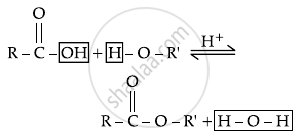
In nucleophilic acyl substitution, the attack of the carbonyl carbon atom of an acyl derivative by nucleophile yields a tetrahedral intermediate. The tetrahedral intermediate then ejects a leaving group. Also, the electron-withdrawing group on carboxylic acid will increase the rate of esterification.
\[\begin{array}{cc}
\phantom{...........}\ce{O}\phantom{.....................}\ce{O}\phantom{}\\
\phantom{...........}||\phantom{.....................}||\phantom{}\\
\ce{\underset{Nucleophilic acyl substitution}{R - OH - R + C - OH -> R - O - C - R}}
\end{array}\]
