Advertisements
Advertisements
Question
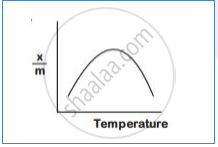
How does chemisorption vary with temperature?
Solution
With an increase in temperature chemisorption first increases as molecules get activation energy for adsorption. After certain chemisorption decreases.temperature,
APPEARS IN
RELATED QUESTIONS
Give reasons for the following observations:
Physisorption decreases with increase in temperature.
Write any two characteristics of Chemisorption.
Why are powdered substances more effective adsorbents than their crystalline forms?
What is the difference between physisorption and chemisorption?
Physisorption is reversible while chemisorption is irreversible. Why ?
Adsorption increases when ____________.
Why is it important to have clean surface in surface studies?
Give an example where physisorption changes to chemisorption with rise in temperature. Explain the reason for change.
Adsorption play no role in:-
Write three differences between Physisorption and Chemisorption.
