Advertisements
Advertisements
Question
How is Methoxyethane prepared from : Methyl iodide
Solution
Methoxyethane, also known as ethyl methyl ether, is an ethyl group with a bonded methoxy. Methoxyethane is a colourless gaseous ether with a medicine-like odour. It is extremely flammable, and its inhalation may cause asphyxiation or dizziness. As a Lewis base, it can react with Lewis acids to form salts and reacts violently with oxidizing agents.
a) Preparation of Dimethyl ether (Methoxymethane) from methyl iodide : When methyl iodide is heated with alcoholic sodium methoxide, it gives dimethyl ether.
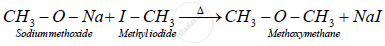
APPEARS IN
RELATED QUESTIONS
Carbon atom in methyl carbocation contains how many pairs of electrons?
(a) 8
(b) 4
(c) 3
(d) 5
How is nitroethane converted into ethylamine.
How is Methoxyethane prepared from : Diazomethane
How is nitroethane converted into N-ethylhydroxylamine
How is nitroethane converted into acetic acid
How can the following conversions be brought about:
Diethyl ether to ethanol
Write chemical reactions of following reagents on methoxyethane:
PCl5
How will you obtain the following? (Give balanced equation.)
Ethyl chloride from diethyl ether.
Convert the following :
anisole to phenol.
