Advertisements
Advertisements
Question
How would you determine the standard electrode potential of the system \[\ce{Mg^{2+} | Mg}\]?
Solution
Set up an electrochemical cell consisting of \[\ce{Mg | MgSO4 (1 M)}\] as one electrode by dipping a magnesium wire in \[\ce{(1 M) MgSO4}\] solution and the standard hydrogen electrode \[\ce{Pt}\], \[\ce{H2 (1 atm) | H+ (1 M)}\] as the second electrode.
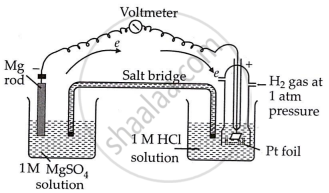
Measure the cell's EMF and note the direction of deflection in the voltmeter. The direction of deflection indicates that electrons flow from the magnesium electrode to the hydrogen electrode, implying that oxidation occurs on the magnesium electrode and reduction on the hydrogen electrode.
Thus, the cell may be represented as follows:
\[\ce{Mg | Mg^{2+} (1 M) || H+ (1 M) | H2 (1 atm), Pt}\]
\[\ce{E^Θ_{cell} = E^Θ_{{H^{+}}/{{H_2}}} - E^Θ_{{Mg^{2+}/Mg}}}\]
Put \[\ce{E^Θ_{{H^{+}}/{{H_2}}}}\] = 0
Hence \[\ce{E^Θ_{{Mg^{2+}/Mg}}}\] = \[\ce{- E^Θ_{cell}}\]
It is observed that the EMF of the cell comes out to be 2.36 V.
Hence, the standard electrode potential for the \[\ce{Mg^{2+} | Mg}\] system will be EΘ = −2.36 V.
APPEARS IN
RELATED QUESTIONS
Consult the table of standard electrode potentials and suggest three substances that can oxidise ferrous ions under suitable conditions.
Calculate the standard cell potential of a galvanic cell in which the following reaction takes place:
\[\ce{2Cr_{(s)} + 3Cd^2+_{( aq)}-> 2Cr^{3+}_{( aq)} + 3Cd}\]
Calculate the ΔrGΘ and equilibrium constant of the reaction.
Account for the following:
E° value for the Mn3+/Mn2+ couple is highly positive (+1.57 V) as compare to Cr3+/Cr2+.
Write the cell reaction and calculate the e.m.f of the following cell at 298 K:
`Sn(s) | Sn^(2+) (0.004 M) || H^(+) (0.020 M) | H_2 (g) ("1 bar") | Pt(s)`
(Given: `E_(Sn^(2+)"/"Sn)^0 = -0.14` V)
In a hydrogen-oxygen fuel cell, combustion of hydrogen occurs to:-
In a galvanic cell, current flows ______
What is the standard free energy change for the following reaction at room temperature? Is the reaction spontaneous?
\[\ce{Sn(s) + 2Cu^{2+}(aq) -> Sn^{2+}(aq) + 2Cu+(s)}\]
Galvanization is applying a coating of ______.
Calculate the standard cell potential of a galvanic cell in which the following reaction takes place:
\[\ce{Fe^2+_{( aq)} + Ag+_{( aq)} -> Fe^3+_{( aq)} + Ag_{(s)}}\]
Calculate the ΔrGθ and equilibrium constant of the reaction.
