Advertisements
Advertisements
Question
Hyperconjugation involves delocalisation of:
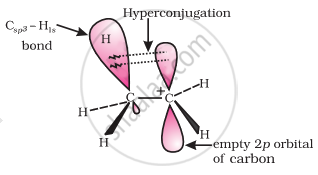
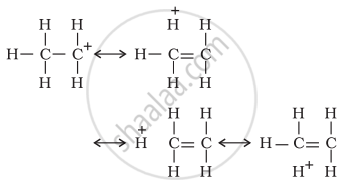
(i) Electrons of carbon-hydrogen σ bond of an alkyl group directly attached to an atom of unsaturated system.
(ii) Electrons of carbon-hydrogen σ bond of alkyl group directly attached to the positively charged carbon atom.
(iii) π-electrons of carbon-carbon bond.
(iv) Lone pair of electrons.
Solution
(i) Electrons of carbon-hydrogen σ bond of an alkyl group directly attached to an atom of unsaturated system.
(ii) Electrons of carbon-hydrogen σ bond of alkyl group directly attached to the positively charged carbon atom.
Explanation:
since hyperconjugation involves the delocalization of electrons of C—H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p-orbital. The electrons of C—H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p-orbital. This is a permanent effect. Hyperconjugation can be shown as-