Advertisements
Advertisements
Question
Identify compound having square pyramidal-shape:from following.
Options
CIF5
BrF5
ICI
BrF3
MCQ
Solution
BrF5
Explanation:
According to VSEPR theory, the shape of BrF5 is square pyramidal and its electron geometry is octahedral because bromine being the central atom has five bonds connected with surrounding fluorine atoms. Each \[\ce{F - Br - F}\] bond making an angle of 90° in the same plane.
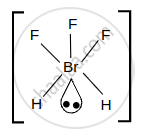
Square pyramidal structure of BrF5
shaalaa.com
Is there an error in this question or solution?
