Advertisements
Advertisements
Question
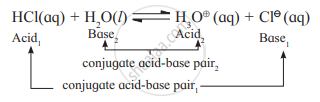
Identify the conjugate acid - base pair in the following reaction.
\[\ce{H2O + HCl -> H3O+ + Cl-}\]
Options
\[\ce{H2O and HCl}\]
\[\ce{Cl- and H2O}\]
\[\ce{H3O+ and Cl-}\]
\[\ce{H3O+ and H2O}\]
MCQ
Solution
\[\ce{H3O+ and Cl-}\]
Explanation:
When base and acid interact, conjugate acid and conjugate base are created.
shaalaa.com
Acids and Bases
Is there an error in this question or solution?
